Our Research interests
Our research interests are in the area of Organic and Organometallic Chemistry. Organometallic chemistry is the study of compounds having metal-carbon bonds. Carbon-containing (organic) molecular fragments that are bonded to metal atoms often have unique geometries and reactivities on account of the metal's electronic properties. Organometallic compounds are important in catalysis, medicine, and the construction of molecular scale devices (nanoscience).
Research goal: Organometallic catalysts
Catalysts have a huge economical impact on all different kind of syntheses.
Catalysts increase the efficiency and selectivity of chemical reactions,
and they help saving ressources and energy. Organometallic compounds often
have unique reactivities on account of the metal's electronic properties.
and they can serve as catalysts in organic transformations. It is our goal to find novel organometallic architectures,
which are catalytically active and easy to handle.
We are interested in
a) the development of novel ligand structures
b) the investigation of relationships between the structure of ligands and the properties of their respective metal complexes
c) the application of these metal complexes as catalysts in organic transformations
d) and how the ligand structures can influence the catalytic activity of the complexes.
We are especially interested in
a) the development of catalytically active iron complexes.
Iron has a number of advantages over other transition metals regularly employed in catalytic
processes: it is cheap, abundant, nontoxic and environmentally friendly. Our approach starts with the synthesis of preformed, well-charcterized iron complexes,
which we subsequently employ in catalysis. Preformed complexes allow for rational ligand design and make mechanistic investigations easier.
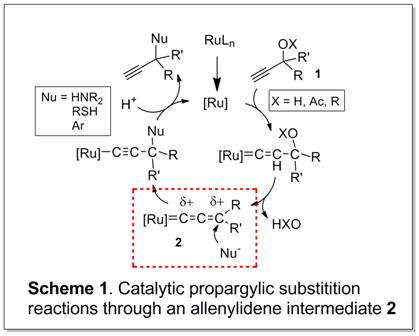
b) the catalytic activation of propargylic alcohols and their derivatives through allenylidene complexes Ru=C=C=CR2.
Certain ruthenium complexes are known to form allenylidene complexes, which can undergo further transformations. Thus, we are also interested in the
synthesis of stable allenylidene complexes, especially chiral ones, which are not very well explored.
Catalytic activation of propargylic alcohols and their derivatives
The nucleophilic substitution of propargyic alcohols and their derivatives (1) is challenging, and can potentially proceed through the mechanism shown in Figure 1. Key intermediate is the allenylidene complex 2, which constitutes an activated version of the propargylic staring material. Our research interests are centered around the properties, stabilities, and the stereochemistry of allenylidene complexes. Chiral allenylidenes and especially allenylidenes with stereogenic metal centers are not very well developed. We also intend to "fine-tune" the electronic properties of potential precursor complexes via the supporting ligands in order to render the allenylidene intermediate reactive in solution.
Synthesis of sterically and electronically tuned ruthenium phosphoramidite complexes and their catalytic applications
We utilize among others phosphoramidite ligands to tune the steric and electronic properties of ruthenium complexes and to establish chirality. Accordingly, we synthesized a series of half sandwich complexes of the general formula [RuCl2(p-cymene)(L)] (3, L = phosphoramidite, Figure 2).
We subsequently determined that all complexes are catalytically active in the formation of
beta-oxo esters (5) from aromatic and aliphatic primary, secondary and tertiary propargylic
alcohols (4) and aromatic and aliphatic carboxylic acids (Scheme 1). Most significantly,
the ruthenium phosphoramidite complex containing an N-benzyl ligand (3c) was catalytically much
more active than the complex bearing N-methyl ligands (3a). Thus, it was established that the
ligand structure can have a profound impact on the catalytic activity of its respective
metal complex.
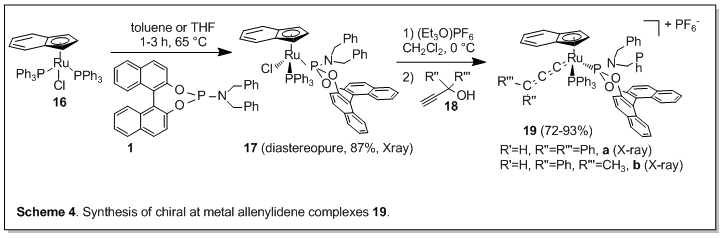
In these experiments, we found that the p-cymene ligand in the ruthenium complexes 3 readily detaches from the metal complex upon heating. We were looking for more robust architectures and were also interested in investigating complexes chiral at the metal. Thus, we synthesized ruthenium complexes of the general formula [RuCl(Cp)PPh3(1)] (Cp = cyclopentadienyl, 6) (Figure 3). Complexes 6b,c were isolated diastereomerically pure and characterized structurally.
The chloride ligand can be removed employing [Et3O]+[PF6]- and the resulting Lewis acidic fragment of the formula [Ru(Cp)PPh3(1)]PF6 is catalytically active in the Mukaiyama aldol reaction (Scheme 2).
Synthesis of the first iron PHOX complexes
To best of our knowledge, iron PHOX chelate complexes have not been reported in the literature and to date have not been applied as catalysts. To perform tuning experiments as described for the phosphoramidite ruthenium complexes, we were interested to determine if iron can form stable PHOX complexes. Thus, we synthesized the first iron PHOX complexes of the general formula [FeCp(CO)(2)]+I- (10) upon heating of the corresponding PHOX ligands with [Fe(Cp)I(CO)2] (Figure 4). The novel iron complexes 10 were characterized structurally, and they show catalytic catalytic activity in oxidation reactions (vide infra).
Synthesis of a series of amino-dithiaphospholane ligands, ruthenium, rhodium and iridium complexes thereof and catalytic applications
Thus far, we have observed steric but no electronic impacts of different phosphoramidite ligands on their catalytic performance (Scheme 1). We were interested to determine whether electronic tuning closer to the phosphorus atom (which coordinates directly to the metal) can influence the catalytic performance of the corresponding metal complexes. Thus, we replaced the two oxygen atoms directly bonded to the phosphorus center in phosphoramidite ligands with sulfur to obtain the new amino-dithiaphospholanes 11a-c (Figure 5). This class of compounds is known, but has to the best of our knowledge never been employed as ligands in transition metal complexes. Accordingly, we successfully employed the amino-dithiaphospholanes 11a-c as ligands in the synthesis of complexes of ruthenium (12), rhodium (13), and iridium (14).
The ruthenium complex 12 was shown to be catalytically active in propargylic substitution reactions (etherification of propargylic alcohols 4 with methanol and ethanol, Scheme 3).
Novel Chiral at Metal Allenylidene Complexes of Ruthenium Obtained with Chirality Transfer
Allenylidene complexes play an important role both as catalysts and intermediates in organic transformations. The ruthenium complexes 17 were obtained from the known ruthenium indenyl precursor 16 in diastereomeric purity (Scheme 4). They could be converted to the allenylidene complexes 19 with chirality transfer, as confirmed by X-ray.
Novel Iron Complexes and their Catalytic Activity in Oxidation Reactions
We have synthesized new phosphoramidite (20, X-ray) and PHOX (21) complexes of iron (Figure 6). They are (as well as the iron complex 10a, Figure 4) catalytically active in the oxidation of activated methylene groups to ketones with t-BuOOH (Table 1).
Novel Chiral Iron PHOX Indenyl Complexes and Their Catalytic Activity in the Mukaiyama Aldol Reaction
We have synthesized new PHOX indenyl complexes of iron (22, X-ray, Figure 7), which are catalytically active in the Mukaiyama aldol reaction according to Scheme 2 (room temperature, 15 minutes, 48-83% isolated yields). We tentatively ascribed the high activity of complex 22 to the indenyl ligand, which is known to enhance the reactivity of metal complexes.
New five-coordinate Ru(II) phosphoramidite complexes and their catalytic activity in propargylic amination reactions
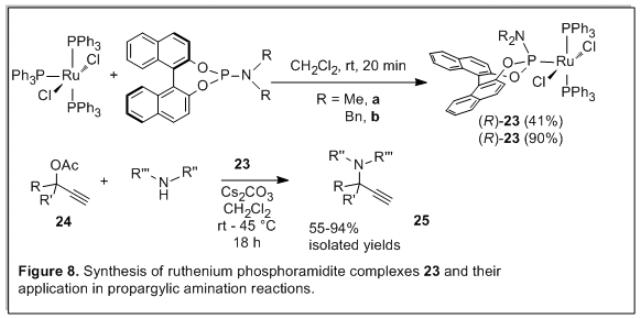
We have synthesized novel ruthenium phosphoramidite complexes [RuCl2(PPh3)2L] (23, Figure 8), where L is a phosphoramiddite ligand. The complexes 23 are much more stable in solution than their precursor complex [RuCl2(PPh3)3]. Complexes 23 catalytically active in the amination of propargylic esters 24 to obtain the propargylic amines 25 (room temperature or 45 degrees, 18 h, 55-94% isolated yields).